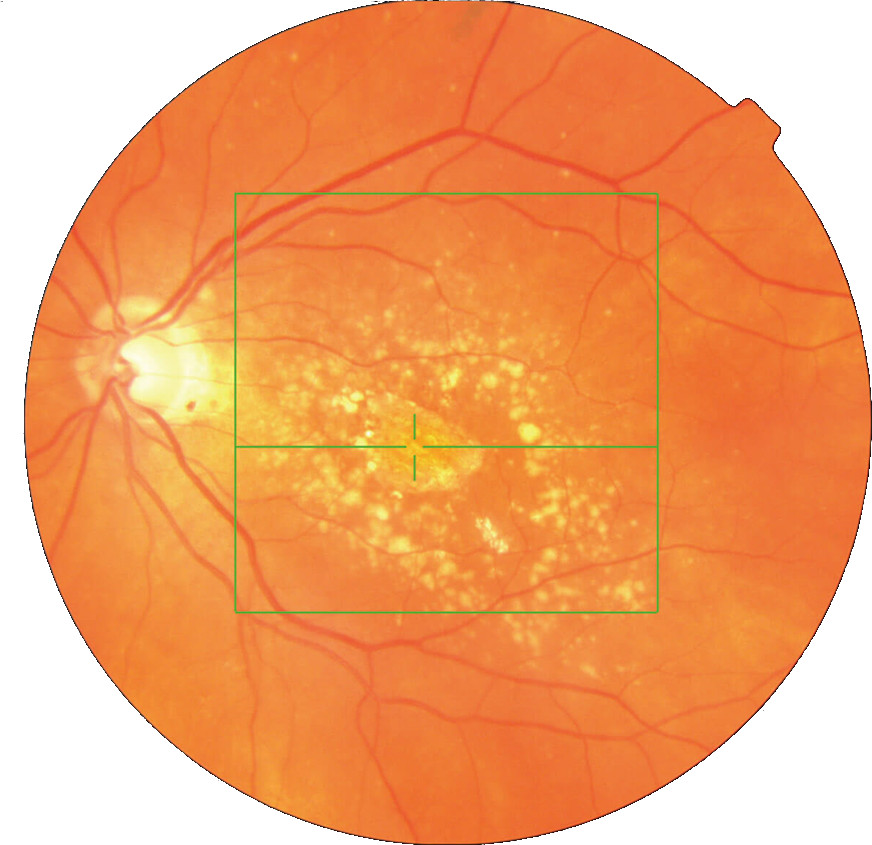
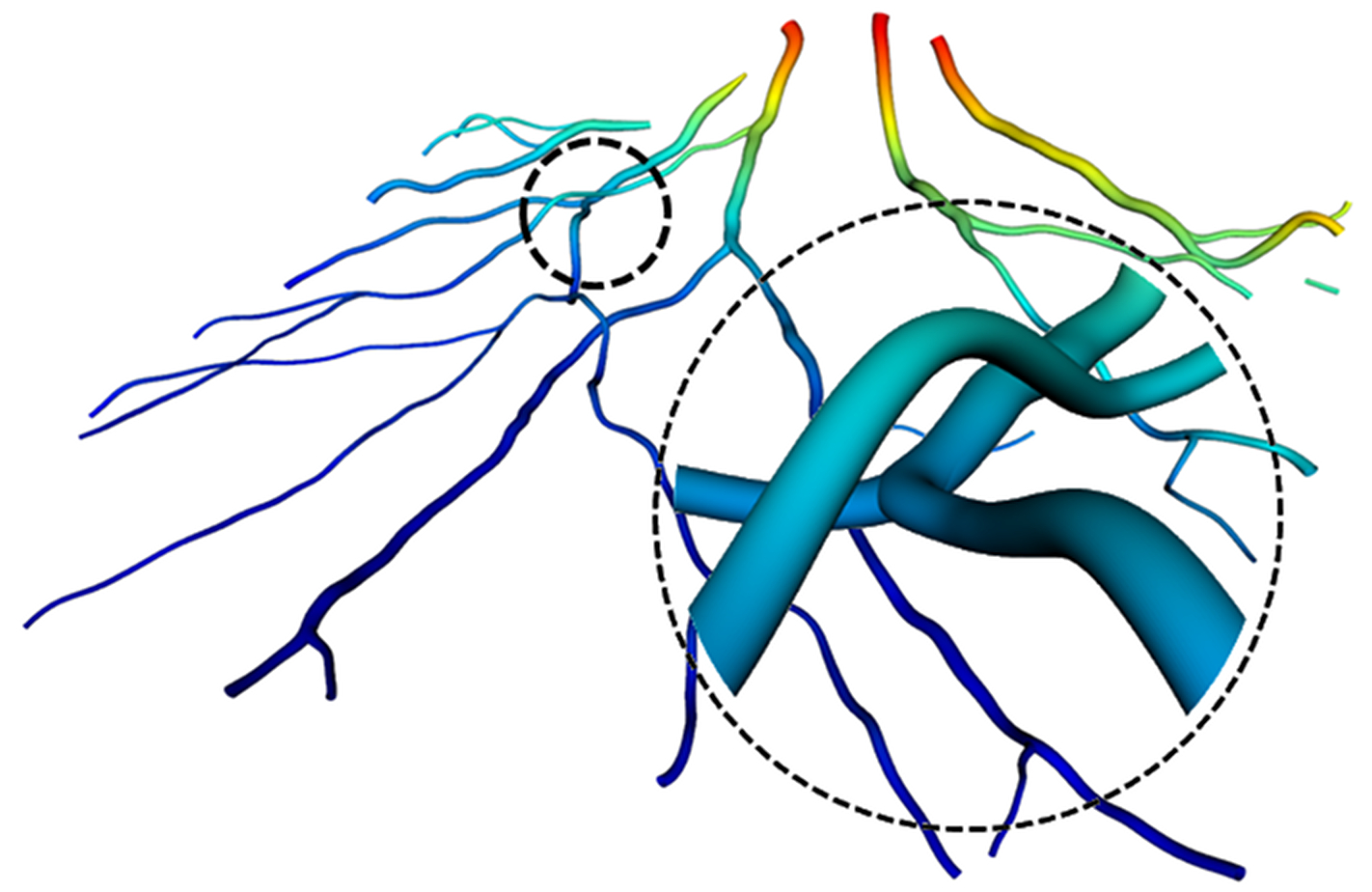
ABOUT US
Research Technology Organisation in the health area dedicated to the development and clinical research of new products for medical therapy and diagnostic imaging.

UFC // Pharmacovigilance Unit of Coimbra
The Pharmacovigilance Unit of Coimbra (UFC) is a Regional Unit of the National Pharmacovigilance System which is supported through a contract with the National Authority of Medicines and Health Products (INFARMED, IP).
+info

IT // Information Technology
The Data Centre is a structure to support Investigator Initiated Research providing Data Management and Electronic Data Capture Solutions.
+info

USGI // Integrated Management System Unit
The Integrated Management System (IMS) has the necessary resources to provide the services and meet the needs and expectations of its Clients and interested parties.
+info
UTT // Translational Research and Technology Transfer Unit
The Translational Research and Technology Transfer Unit is responsible to provide all the support to facilitate and promote the transfer of R&D activities and pre-clinical studies to the development of clinical studies and to enhance the adoptions of best practices in the community.
+info

It is with profound sadness and the deepest respect that AIBILI announces the passing of our beloved Founder and Honorary President, Prof. José Cunha-Vaz. The world has lost a true pioneer in the field of Ophthalmology

AIBILI is pleased to announce the appointment of Daniel Sanches Fernandes as its new Chief Executive Officer, succeeding Cecília Martinho and building on her dedicated leadership and significant contrib


Take part in the 2nd edition of the Technological Showcase “Innovation in Health” and join a privileged platform that brings together research

The AIBILI-DC Research Data Management for Open Science will host a training session titled “Introduction to AIBILI Dataverse Repository.” This online session, aimed at those working in clinical research

Date: 12 September 2025 Location: FMUC – Faculty of Medicine, University of Coimbra Format: In-person or Online We

AIBILI is pleased to announce the upcoming V Research Groups Annual Meeting, scheduled for July 2, 2025. This event will focus on research in ophthalmology and
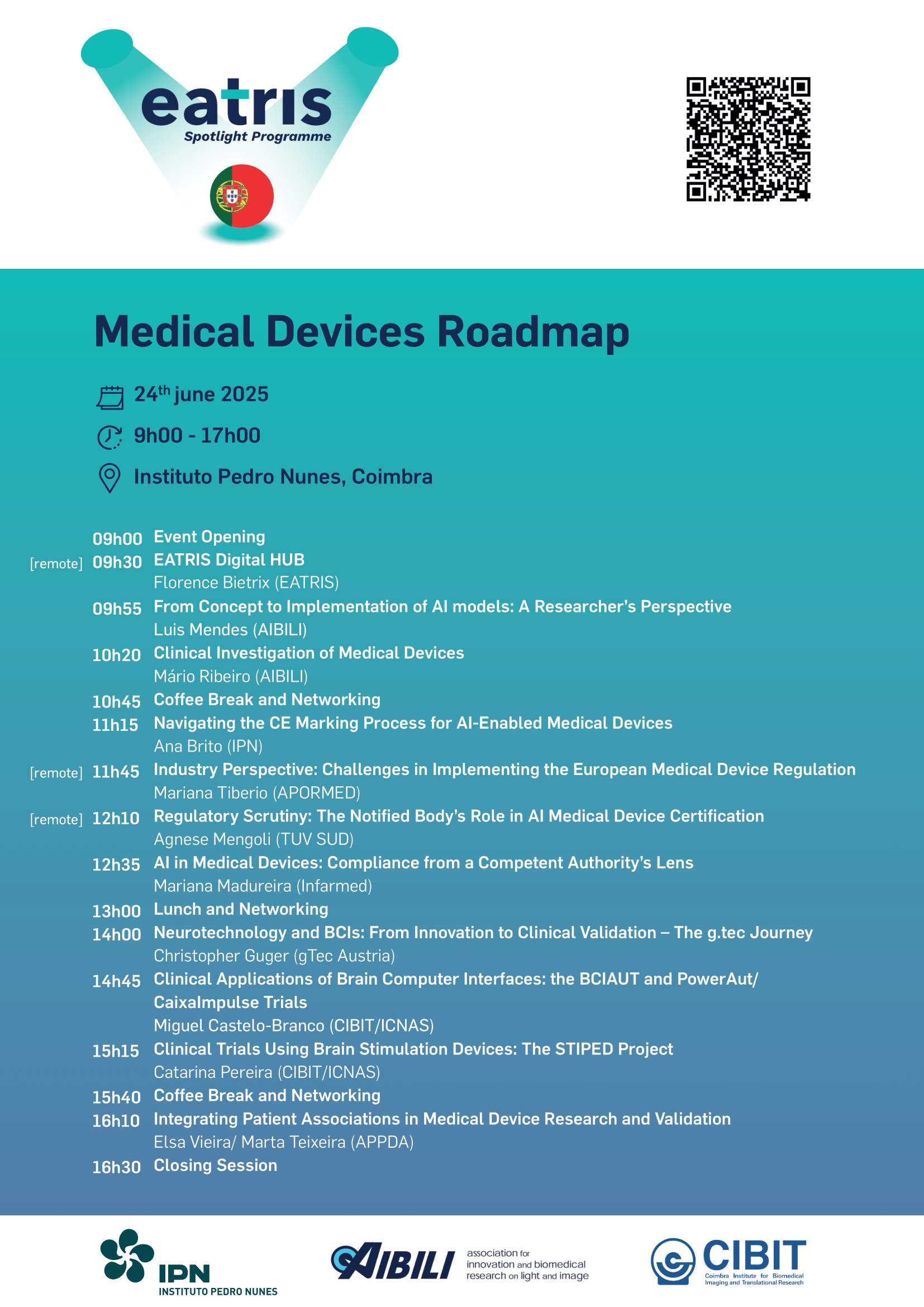
Date: 24 June 2025 Location: Instituto Pedro Nunes, Coimbra Format: In-person or Online We are pleased to announce the Medical Device Roadmap event

On February 19, 2025, the official presentation of the 4th edition of INOVC+ – Intelligent Innovation Ecosystem of the Central Region took place at the Auditorium of the Comissão de Coordenação e Desenvolvimento Reg

The European public-private partnership consortium MACUSTAR .eu has received a third 𝗟𝗲𝘁𝘁𝗲𝗿 𝗼𝗳 𝗦𝘂𝗽𝗽𝗼𝗿𝘁 from

The competition for science &technology-based business ideas is back, recognising innovative national projects with the potential to impact diverse sectors, and applications are open until 2 February. Th

Founded in September 1989, AIBILI began with the mission of supporting technology transfer and translational research in the health sector. Over the years, AIBILI has become a key partner, bridging academic and scientifi

The program for the webinar Data Management for Clinical Research is now available! We invite you to join us on November 12, 2024, from 2:00 PM to 3:00 PM (GMT). Secure your spot and register + info

The program for the AIBILI Open Day is now available! Join us on June 5, 2024, between 2:30 PM and 5:00 PM at FMUC – Auditorium

José Cunha-Vaz, Emeritus Professor of Ophthalmology at the University of Coimbra and Honorary President of AIBILI, has just been awarded the most prestigious global prize in ophthalmology research: the Helen Keller Priz

AIBILI is proud to have developed for the SPO - Portuguese Society of Ophthalmology two registries for Keratoconus and Endophthalmitis. We congratulate SPO for the initiative which will contribute to foster ophthalmology

This week we have received the visit of Prof. António Costa e Silva, the Ministry of Economy and Maritime Affairs. AIBILI Administration had the opportunity to showcase the skills and installed capacities and to reinfo

AIBILI is proud to perform a study on the effectiveness of educational materials available for Vabysmo®, Eylea® and Lucentis®. The MARVEL Study, supported by INFARMED, I.P., is conducted by the Coimbra Regional Pha

Presentations:
- Abnormal retinal fluid in eyes with diabetic center-involved macular edema
- A Conversion Model for OCTA Vessel Density

AIBILI will be presenting at EVOLVE - Digital Transformation Summit 2023 the EVICR.net Eye Platform. This summit will be organised by APDC on October 11, 2023, in Lisbon, Portugal. The + info

AIBILI was present at this ERA4Health workshop - Analysis of bottlenecks and challenges in designing and conducting investigator-initiated multinational clinical studies - through Dr. Cecília Martinho and Dr. Joana Tava

'À Mesa com Saúde' is a health literacy initiative organized by Faculty of Medicine of the Coimbra University and iCBR - Coimbra Institute for Clinical and Biomedical Research, in partnership with the Coimbra School of

AIBILI, as Coordinating Centre of EVICR.net, is organizing the EVICR.net 18th Members Meeting on October 19-20, 2023, in Milan, Italy.

AIBILI will be organising the EAsDEC congress 2023 on June 1-3, 2023 in Coimbra Portugal. Do not miss the opportunity to joint a forum of ophthalmologists and diabetologists aimed at increasing the understanding of di

AIBILI has been recognised as an EATRIS Expert Centre covering areas as regulatory strategy and execution and health technology assessment. EATRIS put out a call for an expression of interest from institutions to prov

Proud to have Patrícia Barreto from AIBILI AMD Research Group receiving the Plácido Prize by the Sociedade Portuguesa de Oftalmologia, during the 65th Portuguese Congress of Ophthalmology. The work entitle

To celebrate the 30 years of INFARMED and for the first time in Portugal the National Pharmacovigilance Day, INFARMED organises on 13/12/2022 a session in Lisbon. AIBILI is one of the Regional Pharmacovigilance Units

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca de novas recomendações no tratamento da síndrome hepatorrenal com medicamentos que contêm terlipressi

Diabetes is a health condition that can affect many parts of the body, including the eyes. Routine eye exams can help identify the early stages of eye problems and protect a person’s vision.

Do not miss AIBILI's speakers at the roundtables on Personalised Medicine (Inês Pereira Marques) and Big Data/Artificial Intelligence (Luis Mendes). This Friday, September 16th during the Technology Exhibition dedica

AIBILI has been recognized as a Technology and Innovation Center. The Technology and Innovation Centers (CTI) are entities dedicated to the production, dissemination and transmission of knowledge, oriented to companies

The Honorary President of AIBILI, Prof. José Cunha-Vaz, was distinguished today, June 9th, in Berlin, Germany, with the Arnall Patz Medal. The Arnall Patz Medal is intended to distinguish outstanding contribu

AIBILI is happy to participate in the Azores Health Summit which will take place on 12-13/05/2022. Our President, Prof. Conceição Lobo, will share AIBILI’s experience in the development and implementation of diabetic
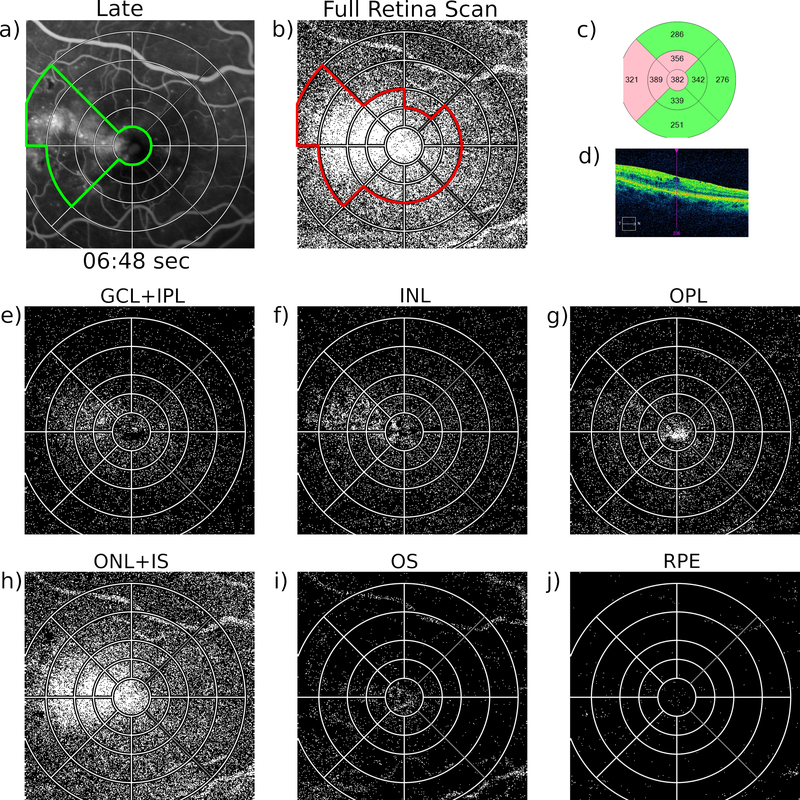
We are honoured to see our recent US Patent published representing another major milestone in AIBILI’s history. This patent US 11,2354,591 B2 follows the EU Patent EP3289565A1 that was received in August 2020. The t

Do not miss the opportunity to hear about AIBILI Data Centre and its relevance for clinical research development. Carlos Domingues, AIBILI DataCentre Director will participate in this conference taking place on May 19

AIBILI has participated through his Projects and Tech Transfer Manager, Daniel Sanches Fernandes, in the EATRIS PORTUGAL Hub Meeting on April 27, 2022 at INFARMED, I.P. in Lisbon.

Congratulations to our Investigators Cláudia Farinha and Patrícia Barreto that were awarded with the Prémio Plácido during the 64th C

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca de novas medidas para minimizar o risco de eventos adversos cardiovasculares major na utilização de Tof

Our honorary president, Prof. José Cunha-Vaz has spoken to the newspaper "Campeão das Provincias" in the scope of the #worldSightDay, celebrated today, October 14! In this communication, Prof. Cunha-Vaz raises the a
We are happy to announce that AIBILI is part of the PTCentroDiH - a regional hub to support small and medium-sized enterprises addressing the digital transformation challenge ahead. PTCentroDiH aims to act a
A AIBILI através do Dr. João Pedro Marques, grader e coordenador médico do CORC, contribui para o estudo inovador PIONEER, publicado na Nature (+ info

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca do risco aumentado de hemorragia pós-parto associada à utilização de medicamentos antidepressivos das

ONLINE COURSE Optical Coherence Tomography in the Posterior Segment - OCT and OCT-angiography April 17, 2021, 8:30 - 13h [GMT] Registration free but mandatory here: + info
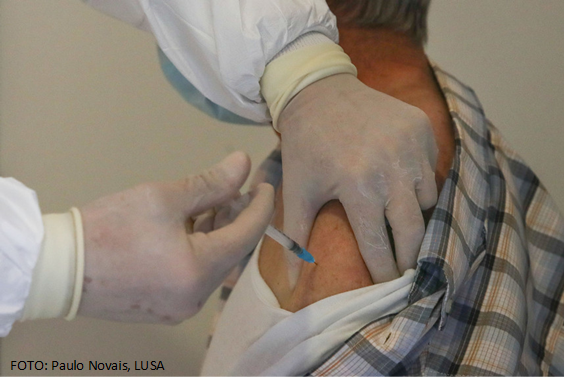
Last March 18, the European Medicines Agency (EMA) announced the selected consortium to monitor possible adverse events to COVID-19 vaccines in Europe for the next 3 years. The consortium, led by the Netherl

We have receive our renewal of Data Centre (DC) certification by ECRIN – European Clinical Research Infrastructure Network! This is an important mark f

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca dos casos de hipertensão grave e síndrome de encefalopatia posterior reversível associada à utilizaç

AIBILI and Pathway Health Consulting are organizing a session focused on Funding and Prices of medicines in Portugal The session will opened by Prof. Francisco Batel-Marques and count with a presentation by
AIBILI researchers Luis Mendes and Sofia Gonçalves will be presenting on the + info

The JoinHealth - Autumn welcome webinar dedicated to Clinical research data will count with the participation of Rita Fernandes and Carlos Domingues from AIBILI - Association for Innovation and Biomedical Research on Lig

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca do risco de aneurisma e dissecção da artéria em doentes sob terapêutica com inibidores do VEGF, quand

AIBILI is proud to announce that our researcher Prof. Rufino Silva was awarded with the 2020 European Society of Retina Specialists (EURETINA) Clinical Research Award with the project “Metabolomics: A Tool for Investig

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca das novas medidas para evitar erros na administração de medicamentos contendo leuprorrelina e relembra-

AIBILI's building has now the name of our Honorary President Prof. José Cunha-Vaz

Don't miss the opportunity to participate in the Workshop dedicated to Good Clinical Practices, organized by CUF Academic and Research Medical Center and AIBILI-Association for Innovation and Biomedical Research on Light

The AIBILI General Assembly met on June 16, 2020, and approved new governing bodies composition as below: General Assembly: Coimbra University - Luís Simões da Silva SUCH –

Alerta de Segurança - Hidroxicloroquina: risco de reações adversas cardíacas em doentes com COVID-19 A hidroxicloroquina está a ser estudada no tratamento de doentes com COVID-19. Os resultados de estudos prelimina

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” destaca-se a evidência acerca da utilização de hidroxicloroquina no tratamento de doentes com COVID-19 e potencial assoc

We would like to inform you that due to the containment measures for the Coronavirus outbreak the normal activity of AIBILI could be affected. However a contingence plan is already in place allowing our collaborators to

O Livro Branco das Doenças Raras é o resultado de um esforço conjunto do Grupo de Trabalho das Doenças Raras da P-BIO e do Centro de Avaliação de Tecnologias de Saúde e Investigação do Medicamento da AIBILI cont

A AIBILI e a ESEnfC assinaram um acordo de parceria para o desenvolvimento de atividades de investigação clínica destas duas instituições da cidade de Coimbra. Encontram, + info

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca de várias questões relacionadas com a segurança de domperidona, nivolumab, carfilzomib, mebutato de in

A CUF e a AIBILI estabeleceram uma parceria para o desenvolvimento de investigação clínica que irá permitir reforçar as competências de ambas as instituições e unir esforços para a concretização de projetos re

The journal Ophthalmic Research has just published a commemorative issue in Honour of Professor José Cunha-Vaz and his achievements in Diabetic Retinopathy, and ground-breaking contribution to basic sci

The 1st Meeting of the Drug Safety and Effectiveness Network (DruSER.net) took place on December 13, 2019. It was an important event to present this network coordinated by CHAD-AIBILI and t

The Centre for Health Technology Assessment and Drug Research of AIBILI will organize on December 13, 2019 the first meeting of the Drug Safety and Effectiveness Research Network. Please check here the p


AIBILI was present at the EATRIS - European Infrastructure for translational Medicine, National Hub Kick off Meeting, at INFARMED, Lisbon. In this meeting, which counted with the presence of EATRIS international repre

Nesta edição do boletim “FARMACOVIGILÂNCIA: Atualizações de segurança de medicamentos” informa-se acerca de várias questões relacionadas com a segurança de metotrexato, fingolimod, montelucaste, terapia horm
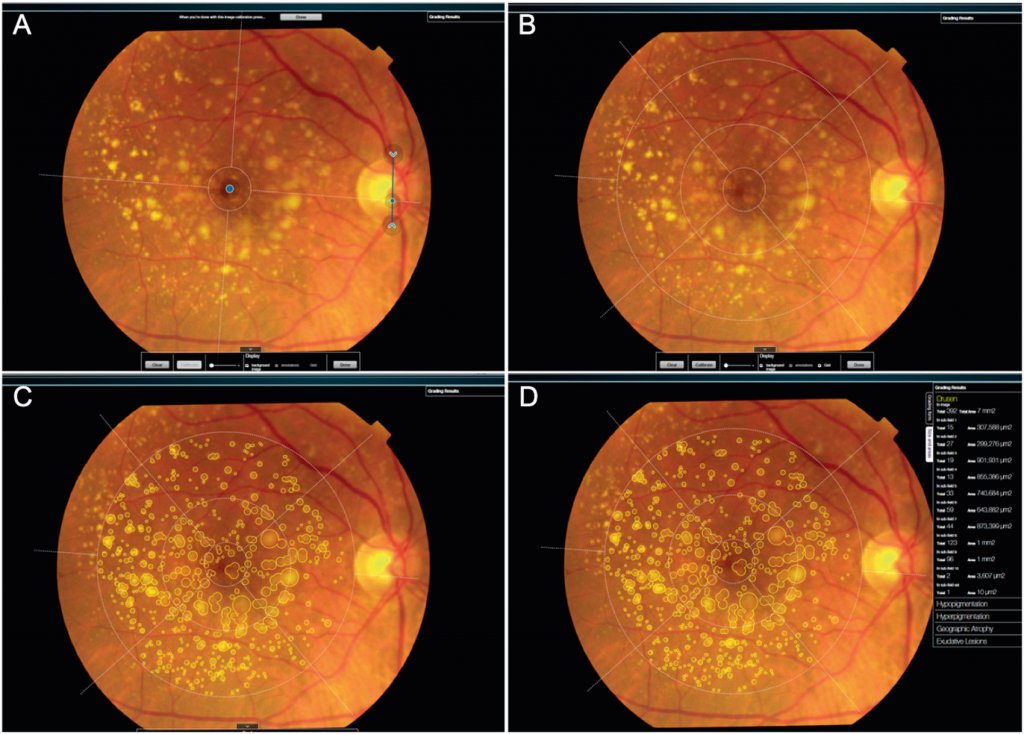
We are pleased to announce our latest publication on RetmarkerAMD® validation, published in the Eye newspaper, a Springer Nature group and official publication of the UK College of Ophthalmologists. By conducting a c

AIBILI was represented by Diogo Mendes at the 19th Annual Meeting of the ISoP International Society of Pharmacovigilance, in Bogotá (Colombia, October 26 to 29).

AIBILI organized in the EVICR.net annual Members Meeting, on October 24 and 25, at Infarmed (Lisbon) that was attended by about 100 participants who had the opportunity to update on the activities in the different subspe

Members of CHAD team were present on the 8th Pharmacovigilance Seminar of Spanish Pharmacovigilance System for Medicinal Products for Human Use, took place in Tordesillas, Spain, on October 23-25. This was the

Farmacovigilância: Atualizações de segurança de medicamentos – Vol. 6, Nº 2 - 2º Trimestre . Consulte: + info

AIBILI was present at TECH@PORTUGAL, on the 4th of July at Alfândega do Porto, an event that presented the best Portuguese innovation and technology. We would like to thank to our partner Retmarker to the support, an

Decorreu no dia 06/06/2019 o Demonstrador Tecnológico AIBILI. Este evento foi organizado em parceria pela Agência Nacional de Inovação (ANI) e a AIBILI, e contará com a presença do Sr. Prof. Eduardo Maldonado, Pres

[video width="1280" height="720" mp4="https://www.aibili.pt/wp-content/uploads/2019/06/Demonstradortecnologico.mp4"][/video] Partilhamos agora o testemunho do Prof. Cunha-Vaz sobre o nosso Demonstrador Tecnológico, r

AIBILI participated in the BIOMEET 2019 to celebrate 20 years of P-BIO. The event took place in Carcavelos on 07/05/2019. Please see here the programme<

Farmacovigilância: Atualizações de segurança de medicamentos – Vol. 6, Nº 1 Pharmacovigilance: Updates on drug safety is a quarterly publication issued by the Pharmacovigilance Unit of Coimbra (UFC) delivering to

A AIBILI recebeu a visita de estudantes de Eng.ª Física e Eng.ª Biomédica da Universidade e Coimbra no dia 19 de Março. Foi efetuada uma apresentação institucional da AIBILI pela Doutora Maria Madeira da UTT, uma

Pharmacovigilance: Updates on drug safety is a quarterly publication issued by the Pharmacovigilance Unit of Coimbra (UFC) delivering to patients and healthcare professionals information on the risks and safe use of medi

AIBILI has co-organized the Onco Pharmacovigilance Conference that was held in Coimbra on 24-25 of January 2019. ONCO Pharmacovigilance Conference is an event to join health professionals in order to address the safety a
CONTACTS
AIBILI
Edifício Prof. Doutor José Cunha-Vaz
Azinhaga Sta. Comba, Celas
3000-548 Coimbra
Portugal
Portugal Geographical coordinates:
40º 13' 06,24" N
8º 24' 54,83" O
Telephone: +351 239 480100
Fax: +351 239 480117