We are happy to announce that AIBILI is part of the PTCentroDiH – a regional hub to support small and medium-sized enterprises addressing the digital transformation challenge ahead.
PTCentroDiH aims to act as a one-stop-shop to foster competitiveness, innovation and territorial cohesion in the Centro Region of Portugal. Among 21 entities AIBILI will specifically contribute with cybersecurity and artificial intelligence services.
AIBILI has a Data Centre certified by ECRIN (unique in the Iberian Peninsula certified by this standard) that gives PTCentroDiH a strategic position in clinical research, and data management in accordance with the regulations.
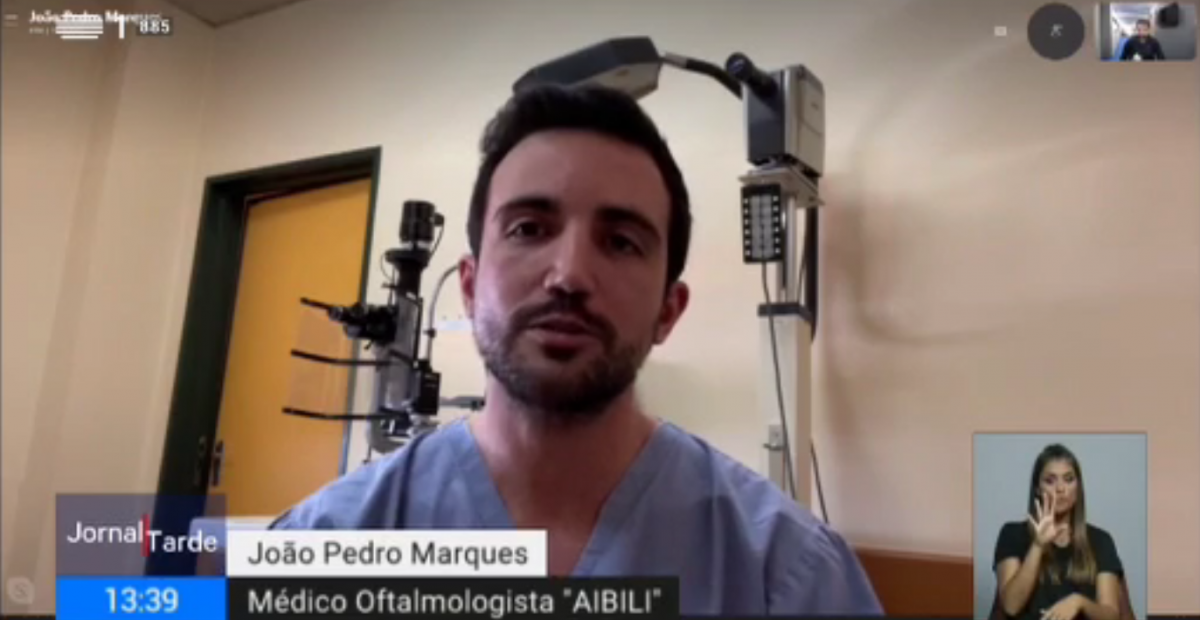
AIBILI has also extensive experience in algorithms’ development, which that allows the automatic detection of injuries/ anomalous conditions in ophthalmic images, as well as in the development of projects within the scope of artificial intelligence.
For more information please visit www.ptcentrodih.pt