CEC // Clinical Trials Centre
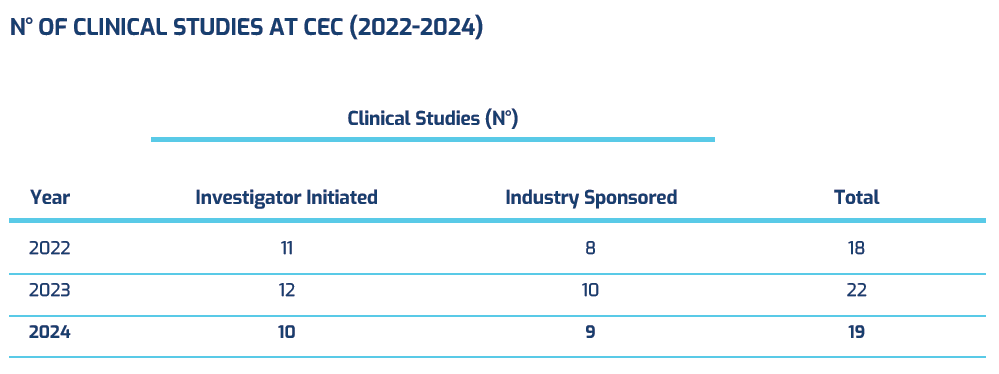
Investigator Initiated Studies
Diabetic Retinopathy
- CORDIS – Characterization of retinal vascular disease in eyes with mild to moderate Nonproliferative diabetic retinopathy in Diabetes type 2, using novel non-invasive imaging methods, in a longitudinal and prospective clinical study with 2 years of duration
ClinicalTrials.gov nº: NCT03696810
Financial support: Portugal 2020 – 02/SAICT/2017 – Project nº 030375
- RECOGNISED – Retinal and Cognitive Dysfunction in Type 2 Diabetes: Unraveling the Common Pathways and Identification of Patients at Risk of Dementia
ClinicalTrials.gov nº: NCT04281186
Financial support: European Union – H2020-SC1-BHC-01-2019-847749
- PROGRESS 10 – Progression of Diabetic Retinopathy. Identification of Signs and Surrogate outcomes -10-year follow-up
ClinicalTrials.gov nº: NCT04650165
- CHART – Characterization of Retinal disease progression in eyes with NPDR in diabetes Type 2 using non-invasive procedures
ClinicalTrials.gov nº: NCT04636307
Financial support: IIR Grant from Bayer
- RICHARD – Retinal Ischemia characterization in diabetes
ClinicalTrials.gov nº: NCT05112445
Financial support: IIR Grant from Boehringer Ingelheim
- Exploratory project – Diabetic Retinopathy: from clinical to cellular phenotyping
Financial support: AIBILI
- PREDICTION – Prediction of Retinal Ischemia in Diabetes
ClinicalTrials.gov nº: NCT05581225
Age-Related Macular Degeneration
- MACUSTAR – Intermediate AMD: Development of novel clinical endpoints for clinical trials in patients with a regulatory and patient access intention
ClinicalTrials.gov nº: NCT03349801
Financial Support: European Union and EFPIA – Innovative Medicines Initiative 2 Joint Undertaking – Grant Agreement nº 116076
- AMDMetab – Metabolomics: An Integrative Tool for Investigating the Pathogenesis of Age-related Macular Degeneration
Partner: Massachusetts Eye and Ear Infirmary (MEEI) and the Harvard Medical School, Boston, USA
ClinicalTrials.gov nº: NCT04241536
Financial Support: EURETINA Clinical Research Award
Retinal Degenerative Diseases
- STAR – Development of a Model for Advanced Screening for Timely Treatment of Age-Related Eye Diseases
- EYEMARKER – Characterization of potential biomarkers of Eye Disease and Vision Loss
ClinicalTrials.gov nº: NCT02500862
Industry Sponsored Clinical Trials
Diabetic Macular Edema
-
- A two-year, three-arm, randomized, double-masked, multicentre, phase III study assessing the efficacy and safety of brolucizumab versus aflibercept in adult patients with visual impairment due to diabetic macular edema (KESTREL)
EudraCT nº: 2017-004742-23
- A Multicenter, Open-Label Extension Study To Evaluate The Long-Term Safety And Tolerability Of Faricimab In Patients With Diabetic Macular Edema (Rhone-X)
EudraCT nº: 2020-000402-29
- A Phase 2 Randomized, Placebo-controlled, Double-masked Proof-of-concept Study to Investigate the Efficacy and Safety of Runcaciguat (BAY 1101042) in Patients With Moderately Severe to Severe Non-proliferative Diabetic Retinopathy (NEON)
EudraCT nº: 2020-002333-15
Age-Related Macular Degeneration
- A 52-week multicenter, randomized, double-masked, 2-arm parallel study to compare efficacy, safety and immunogenicity of SOK583A1 to Eylea®, administered intravitreally, in patients with neovascular age-related macular degeneration (Mylight)
EudraCT nº: 2019-004838-41
Glaucoma
- Long-Term Surveillance Study of Latanoprost to Monitor Hyperpigmentation changes in the eye in Pediatric Populations (A6111144)
Neurological Disorders
- A multicenter, randomized, double-blind, parallel-group, placebo-controlled variable treatment duration study evaluating the efficacy and safety of Siponimod (BAF312) in patients with secondary progressive multiple sclerosis (EXPAND)
EudraCT nº: 2012-003056-36
- Multicenter, non-comparative extension to study AC-058B301, to investigate the long-term safety, tolerability, and control of disease of ponesimod 20 mg in subjects with relapsing multiple sclerosis (OPTIMUM)
EudraCT nº: 2016-004719-10
Oncology
- A Phase 3 Multicenter, Open-Label, Randomized, Controlled Study of Oral Infigratinib Versus Gemcitabine With Cisplatin in Subjects With Advanced/Metastatic or Inoperable Cholangiocarcinoma With FGFR2 Gene Fusions/Translocations: the PROOF Trial (PROOF)
EudraCT nº: 2018-004004-19

