PROJECT NAME
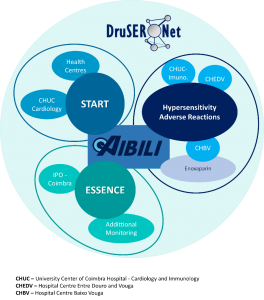
CHAD
- ‣ START-Portugal-Register: The PorTuguese Survey on anTicoagulated pAtients RegisTer
- ‣ ESSENCE – The Effectiveness, Safety and Patient Experience on Oncology Treatments: Achieving Real-World Evidence
- ‣ Rede DruSER.Net – Drug Safety and Effectiveness Network